Model No.︰MED ABS M203FC
Brand Name︰medical abs resin
Country of Origin︰China
Unit Price︰-
Minimum Order︰25 KG
Medical grade ABS resin
ISO10993-5,-10,
FDA,
ISO22196
| Type | Color No. | Mat No. | Oriign | Available |
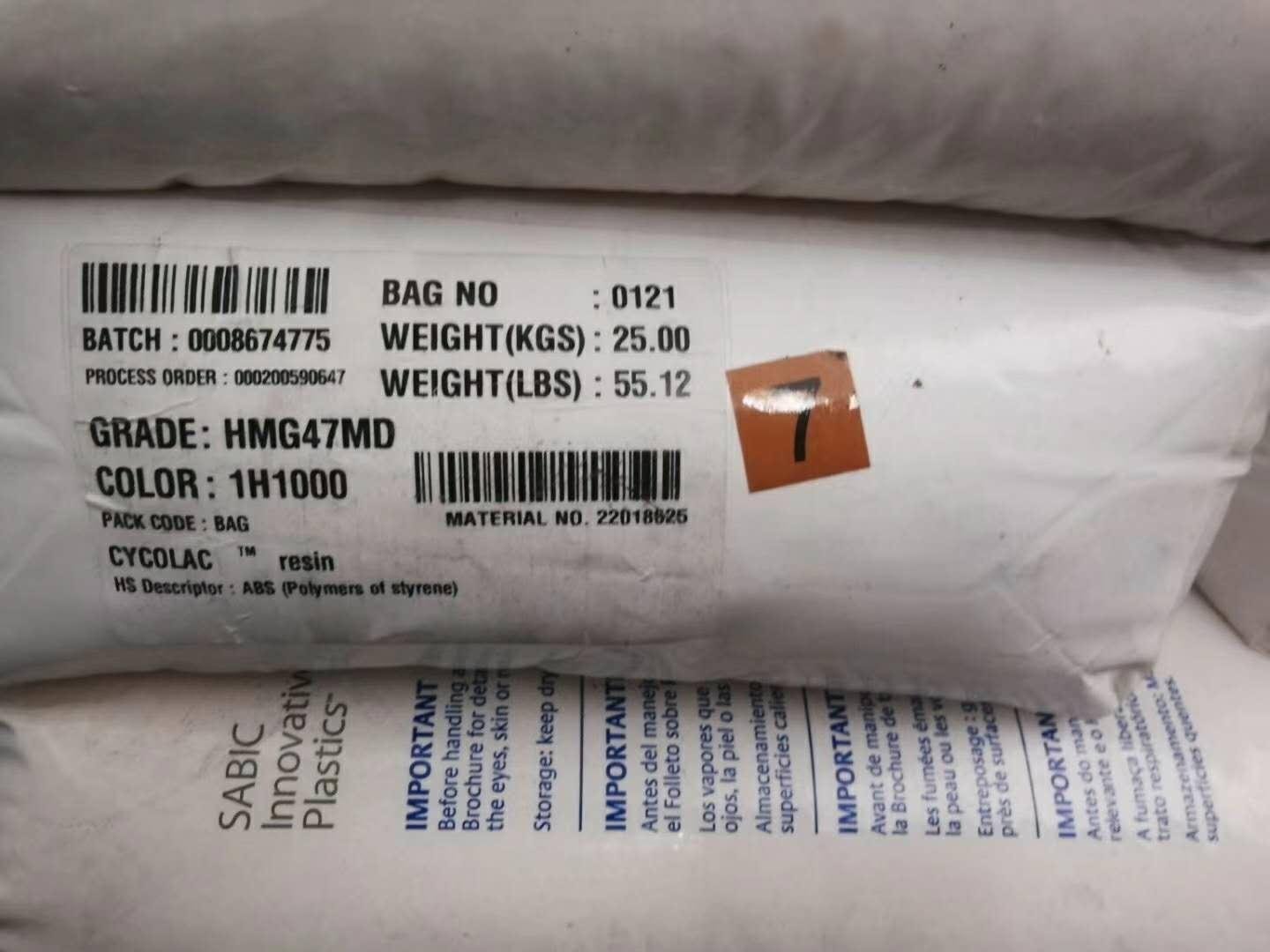
| CYCOLAC HMG47MD |
1H1000 8H4D025 |
22018625 -55lbs/bag |
Canada USA |
on request |
| CYCOLAC HMG94MD | 1H1000 | 22023443 | USA | 1,000 kg |
| MAGNUM 8391MED | N/A | N/A | USA | on request |
|
Antimicrobl ABS LM915NB |
NP White |
ISO22196 ISO10993-5,-10,-23 ASTM G21 FDA 21CFR 181.32 |
Korea | on request |
| Lustran H950 | 901510 black | HDT 105℃ in 1.8Mpa | EU | 500 kg |
| LUSTRAN 348 |
000000 natural
012002 snow-white (50020904) |
Meets FDA modified ISO 10993-1 requirements Meets U.S. Pharmacopeia 23 Class VI test requirements. APPLICATIONS:Components of intravenous (IV)systems,Surgical instruments Diagnostic test kits |
USA |
2 box
(726kg/box)
3 box |
| NOVODUR M203FC | NR | 50022637 | Germany | 20 kg |
| ELIX ABS M203FC | 000000 | 99004132 | SPAIN | 1,400 kg |
|
**Notes: Our inventory list changes every month, Any compounding more color for medical ABS application, Please send e-mail to : x.g.chiang@gmail.com |
| Lustran |
Payment Terms︰ T/T
Packing︰ 25KG/BAG
Lead Time︰ 30 DAYS